Wasserburg, Germany

- Established
- 2010 (acquired)
- Size
- 344,450 ft2
- Employees
- ~400
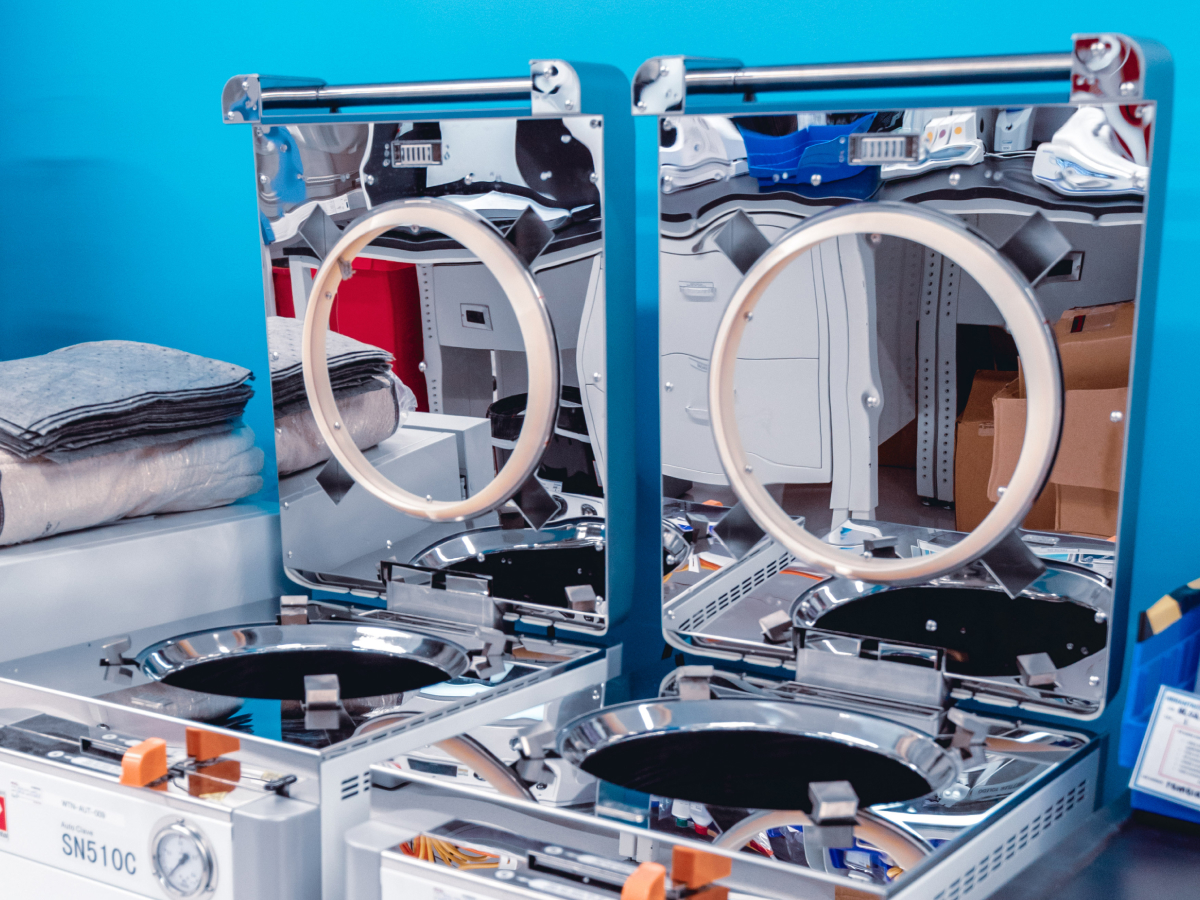
- Leading sterile manufacturing facility located south of Munich, Germany
- Lyophilization Center of Excellence
- Fills in vials with highly automated, state-of-the-art equipment
- Small run, aseptic liquid filling into vials or ampoules
- Ranging vial and ampoule sizes
- Flexible batch sizes
- Multi-product facility
Key Attributes
- Leading sterile manufacturing facility located south of Munich, Germany
- Lyophilization Center of Excellence
- Fills in vials with highly automated, state-of-the-art equipment
- Small run, aseptic liquid filling into vials or ampoules
- Ranging vial and ampoule sizes
- Flexible batch sizes
- Multi-product facility
Facility Highlights
- Approved by the local German Government (EU-GMP), Russia, US FDA, KFDA, TGA, GCC-DR, HPB Health Canada, Japan, ANVISA, and the Drug and Medical Device Institute of Turkey
- ISO 14001, ISO 45001, ISO 50001 certified