Recimagine™ Continuous Production System

The Future of xRNA Manufacturing
Recimagine™ Continuous Production System (CPS) is an intelligent, end-to-end continuous manufacturing platform that has already demonstrated a ~76% reduction in RNA production timelines, with a future target of single-day manufacturing.
Built for scalability, Recimagine CPS spans from process development runs to GMP- production of up to 40 g/day. This seamless pathway ensures continuity from lab bench to commercial readiness, transforming how advanced therapies are developed and delivered.
The Building Blocks of Accessible xRNA Therapeutics
In 2022, average cost of a biological product, $174 per prescription, was 3.7 times greater than average cost of a small molecule drug, $48 per prescription1. While the specific numbers differ slightly in other markets worldwide, the challenge remains: The promise of advanced biologics is inaccessible to many patients due to their expense.
Recimagine CPS directly addresses these challenges by combining digital process development, integrated continuous manufacturing, the Recimagine PAT system, and a continuously expanding Knowledge Hub. Together, these components accelerate development, reduce costs, and increase global accessibility for advanced therapies.

Digital process development
High-throughput screening and simulation replace most trial-and-error DOE work, saving an estimated $150,000–$250,000 per avoided run while providing optimized digital recipes for faster scale-up.
Traditional trial-and-error DOE is open-ended and offers limited visibility into costs and timelines. Using the Recimagine Simulator with high-throughput screening libraries can reduce process development costs by over $150k per run.
Continuous integrated manufacturing
Nested batch integrated manufacturing, coupled with PAT-driven real time release allows for continuous flow of material from upstream reactions to fill finish.
Process models inform PAT-driven controls, while inline analytics monitor each stage in real time to ensure critical quality attributes (CQAs) are achieved consistently.
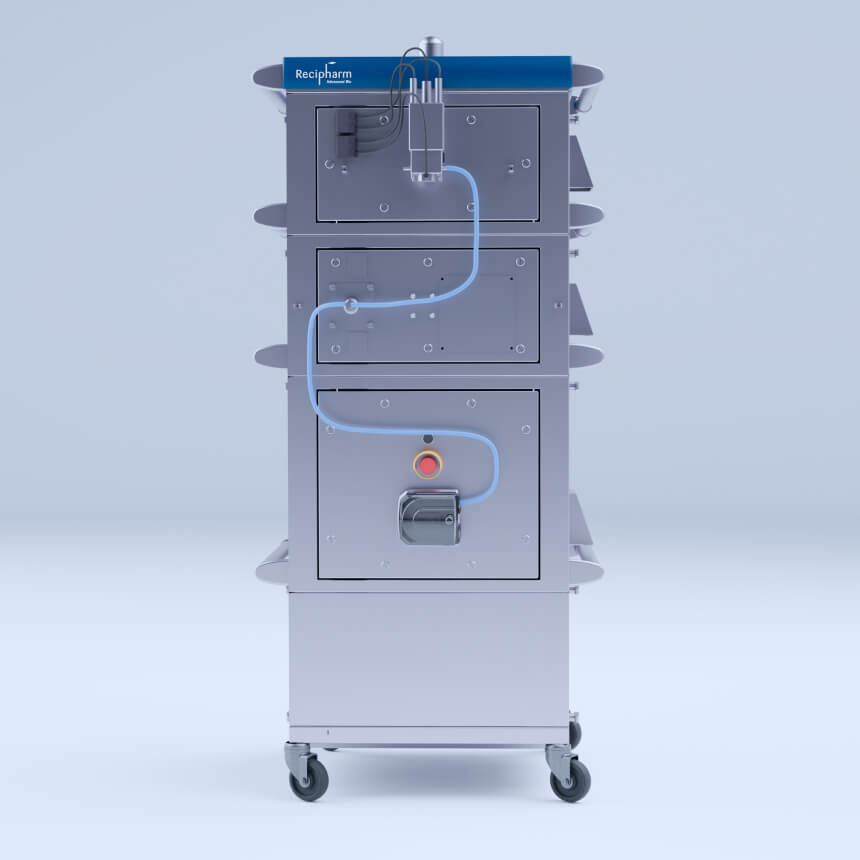
Process analytical technologies
Recimagine PAT’s inline process monitoring replaces offline QC testing; eliminating the stop/start gating that encumbers traditional batch manufacturing. PAT provides equivalent assay support without sacrificing speed.

Knowledge Hub
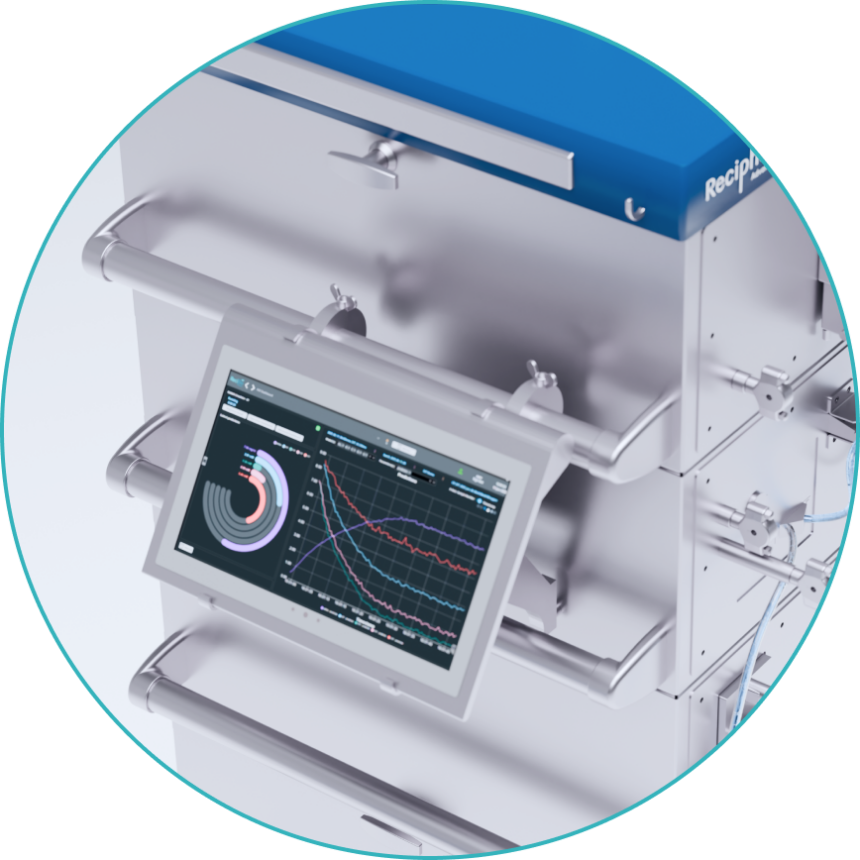
A centralized, AI-enabled repository that grows smarter with every run. By capturing and learning from process data, the Knowledge Hub drives predictive control strategies and improves experimental design over time.
The Future of Advanced Therapy Manufacturing Is Fast
Recimagine CPS combines extensive scientific expertise, AI-enabled technologies, and state-of-the-art process analytic technologies (PAT) to reduce the finished drug product timeline by up to 67%.
Integrated with Simulator and PAT
Recimagine CPS is the manufacturing backbone of the Recimagine ecosystem. Digital recipes generated in the Simulator flow directly into CPS, enabling faster process transfer and scale-up. Inline monitoring from Recimagine PAT provides real-time assurance that every stage meets critical quality attributes (CQAs). By uniting simulation, analytics, and production, Recimagine CPS delivers an intelligent, end-to-end solution for advanced therapy manufacturing.
Recimagine™ Continuous Production System
Intelligent, end-to-end continuous manufacturing of xRNA therapeutics and vaccines, coupled with PAT-driven real-time release, allows for the continuous flow of material from upstream reactions to fill finish, ultimately decreasing production time from months to days.
Learn MoreRecimagine™ Continuous Production System
Intelligent, end-to-end continuous manufacturing of xRNA therapeutics and vaccines, coupled with PAT-driven real-time release, allows for the continuous flow of material from upstream reactions to fill finish, ultimately decreasing production time from months to days.
Learn More
Recimagine™ Simulator
A virtual process development allows teams to design, test, and refine manufacturing strategies entirely in silico before committing resources to the lab.
Learn MoreRecimagine™ Simulator
A virtual process development allows teams to design, test, and refine manufacturing strategies entirely in silico before committing resources to the lab.
Learn More
Recimagine™ PAT
Universal, modular, multi-attribute analytics skid that acts as “QC on wheels.” Recimagine PAT is more than a testing tool; it provides the digital backbone for intelligent manufacturing.
Learn MoreRecimagine™ PAT
Universal, modular, multi-attribute analytics skid that acts as “QC on wheels.” Recimagine PAT is more than a testing tool; it provides the digital backbone for intelligent manufacturing.
Learn More
Consolidated xRNA Processing Solutions
Recipharm Advanced Bio provides comprehensive xRNA manufacturing solutions under one roof, including plasmid DNA and lipid nanoparticle (LNP) production.
Our in-house vector design and process development expertise delivers consistently high-quality final pDNA products.
Learn MoreOur facilities and equipment are designed for every phase of your project, scale from 1 to 50g, and utilize a range of mixing technologies
Discover CapabilitiesFeatured Innovations & Collaborations
xRNA Manufacturing Resources

Transform Development and Manufacturing with Recimagine™
The Recimagine ecosystem tackles the toughest challenges in therapeutic production, including high costs, inefficient development, and inconsistent quality. Together, we can reimagine how life-changing therapies are made and deliver them to patients faster than ever before. Partner with us to accelerate what’s next.